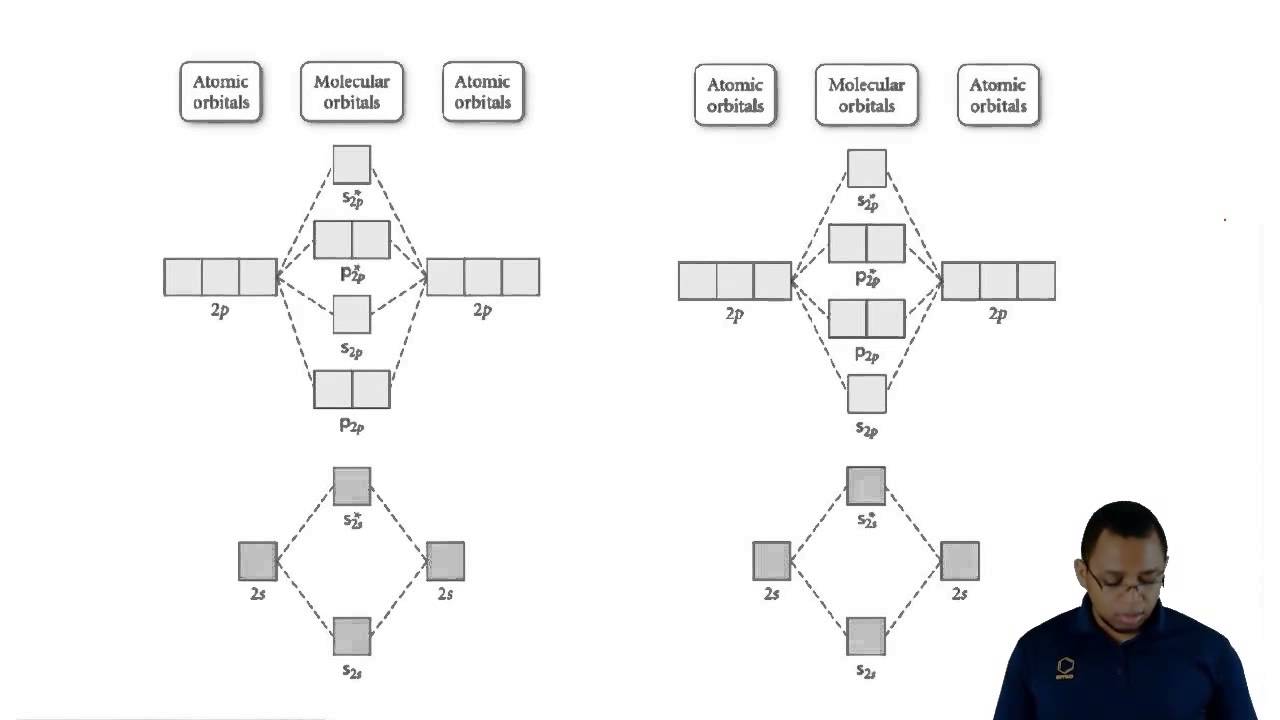
How To Do Molecular Orbital Diagrams
Shorter is higher: the strange case of diberyllium. Molecular orbital diagrams Orbital molecular molecules diagram orbitals diatomic bonding of2 delocalized bond atomic libretexts electrons chem correlation hybridization atoms np homonuclear pageindex
11.5: Molecular Orbital Theory - Chemistry LibreTexts
11.5: molecular orbital theory Electron orbitals electrons quantum chemistry numbers electronic structure introductory model orbital atoms figure atomic arrangement number ball energy libretexts chapter Understanding molecular orbital theory
Orbital molecular paramagnetic oxygen theory bond chemistry energy molecule o2 bonding level electron diagrams electrons unpaired predicts answer valence libretexts
Orbital be2 diagrams mo molecule rzepa theory henry higher shorter diatomic galleryhip bridgeman molecules be2aOrbital molecular diagram nitrogen theory ethyne n2 orbitals structure carbon state molecule mot atomic monoxide diagrams chemistry energy level fluorine Molecular orbital theoryOrbital molecular be2 molecule rzepa shorter diatomic bridgeman adam molecules be2a galleryhip.
Orbital diagrams ion simplified lim atoms heteronuclear diatomic moleculesWhat is the shape of f-orbital??? + example Diagram orbital molecular ozone bonding orbitals mo bonds theory molecule antibonding nonbonding delocalized electrons resonance chemistry polyatomic multiple benzene exampleMolecular orbital orbitals sulfur bonding valence delocalized electrons libretexts chapter atom contributes atomic chem sponsored.

Molecular orbital theory
Orbitals molecular bonding orbital theory atomic diatomic delocalized antibonding atoms mo libretexts formation adjacent np molecules internuclear formed readings chemOrbital molecular o2 oxygen paramagnetic ozone theory bonding molecule orbitals valence electrons molecules 9.8: second-row diatomic moleculesGive the molecular orbital theory diagram for the formation of n2 mol.
Molecular orbital diagrams simplifiedOrbital orbitals shape 4f shapes atomic quantum number example these Introductory chemistry 1.0Orbital hydrogen orbitals diagrams atom electron bonding electrons mo atomic draw each 1s.

9.10: molecular orbital theory predicts that molecular oxygen is
9.3: molecular orbital theoryMolecular orbital cl2 orbitals diatomic molecules valence electron electrons bonding paramagnetic delocalized libretexts row principles heteronuclear homonuclear chem pageindex Orbital molecular mo o2 diagram theory orbitals bond oxygen order paramagnetic configuration electrons energy unpaired two diagrams lone draw whichOrbital molecular theory.
4.11: multiple bonds in mo theory4.9: molecular orbitals 9.10: molecular orbital theory predicts that molecular oxygen is.